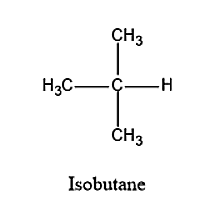
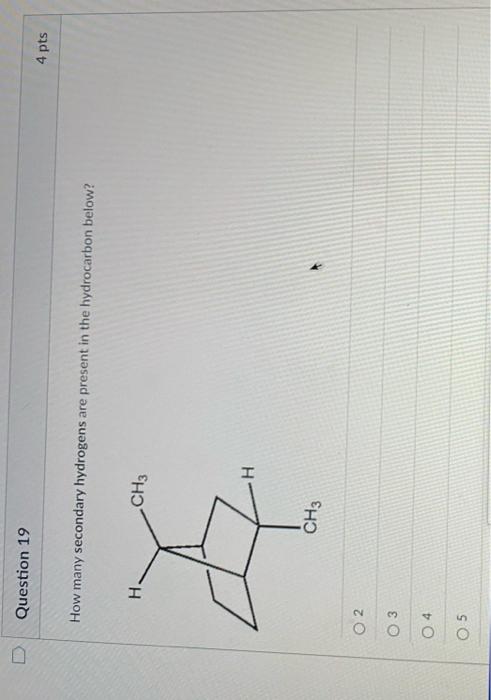
Science Chemistry How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 16 2 6. 7. How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 16 2 6. 7. Chemistry: Matter and Change 1st Edition ISBN: 9780078746376 Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Organic Chemistry: Alkanes and Halogenated Hydrocarbons
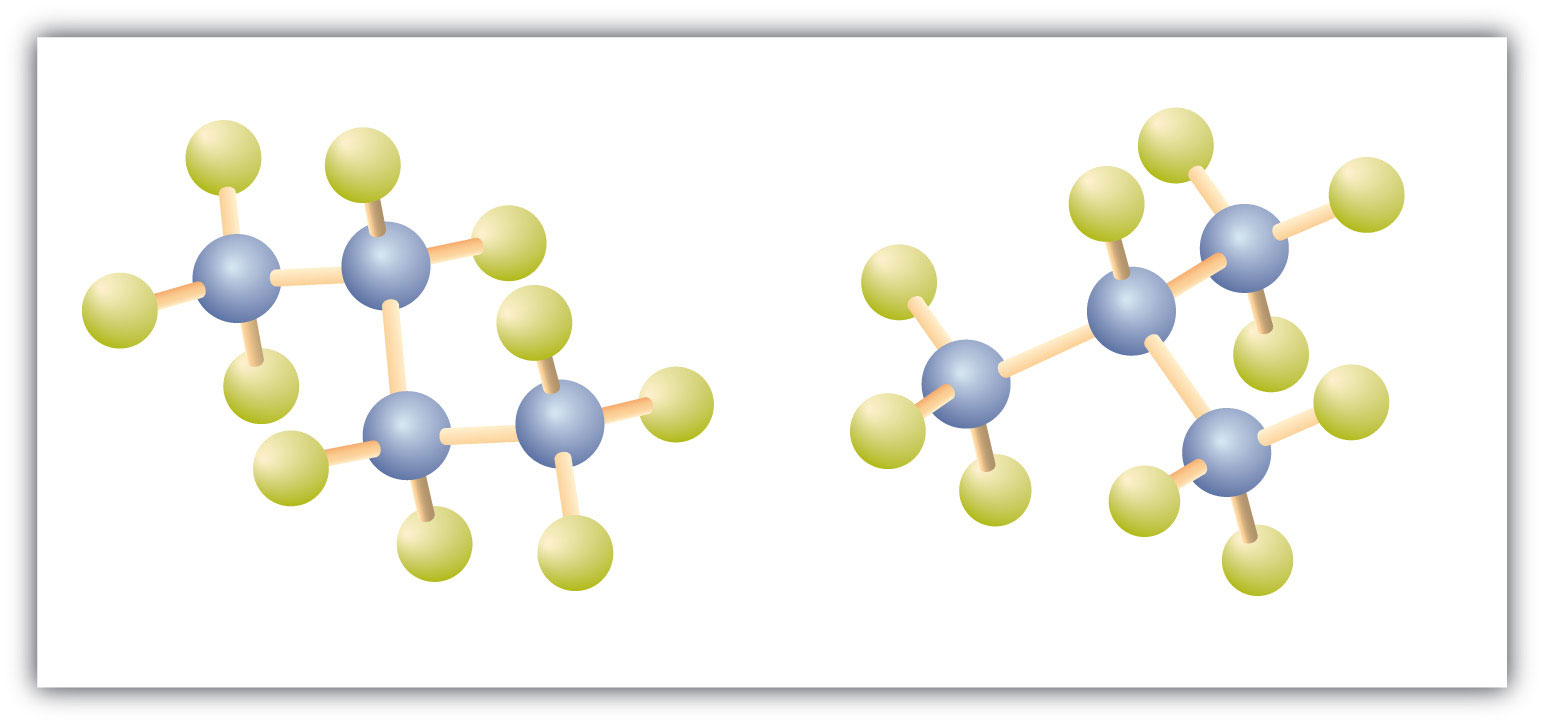
Oct 27, 2022This compound contains 16 hydrogen atoms for a molecular formula of C 8 H 16. Location of the hydrogen atoms: In this figure a ring composed of four C H subscript 2 groups and one C H group in a pentagonal shape is shown. From the C H group, which is at the right side of the pentagon, a C H is bonded.

Source Image: pearson.com
Download Image
Solution for How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 8 16 6. 7. Skip to main content. close. Start your trial now! First week only $4.99! arrow … How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 8 16 6. 7. BUY. Chemistry: Matter and Change. 1st Edition.
Source Image: vedantu.com
Download Image
in the given compound : Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4 ), carbon forms covalent bonds with four hydrogen atoms. Each bond corresponds to a pair of shared electrons (one from carbon and one from hydrogen), giving carbon the eight electrons it needs for a full outer shell.

Source Image: chegg.com
Download Image
How Many Secondary Hydrogens Are Present In The Hydrocarbon Below
Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4 ), carbon forms covalent bonds with four hydrogen atoms. Each bond corresponds to a pair of shared electrons (one from carbon and one from hydrogen), giving carbon the eight electrons it needs for a full outer shell. Oct 12, 2023The term ‘ secondary hydrogens ‘ generally refers to hydrogen atoms that are attached to secondary carbon atoms, which, in this case, are those attached to two other carbon atoms. The molecular formula of this compound is C8H16.
Solved 9) How many secondary hydrogens are present in the | Chegg.com
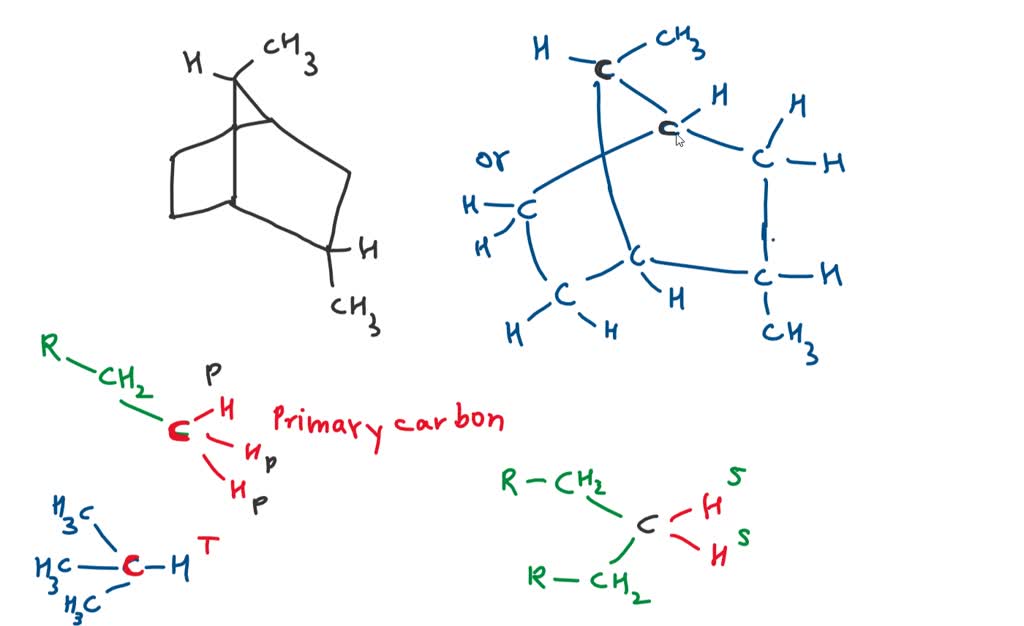
36) How many tertiary hydrogens are present in the hydrocarbon below? A) 2 B) 3 C) 4 D) 1 E) 5 This page titled 1.5: Practice Questions for Chapter 4 is shared under a not declared license and was authored, remixed, and/or curated by Sergio Cortes . Primary secondary tertiary hydrogens in hydrocarbon – Brainly.in

Source Image: brainly.in
Download Image
Organic Compounds 36) How many tertiary hydrogens are present in the hydrocarbon below? A) 2 B) 3 C) 4 D) 1 E) 5 This page titled 1.5: Practice Questions for Chapter 4 is shared under a not declared license and was authored, remixed, and/or curated by Sergio Cortes .
Source Image: 2012books.lardbucket.org
Download Image
Organic Chemistry: Alkanes and Halogenated Hydrocarbons Science Chemistry How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 16 2 6. 7. How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 16 2 6. 7. Chemistry: Matter and Change 1st Edition ISBN: 9780078746376 Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Source Image: saylordotorg.github.io
Download Image
in the given compound : Solution for How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 8 16 6. 7. Skip to main content. close. Start your trial now! First week only $4.99! arrow … How many secondary hydrogens are present in the hydrocarbon below? H. CH3 CH3 8 16 6. 7. BUY. Chemistry: Matter and Change. 1st Edition.

Source Image: byjus.com
Download Image
SOLVED: How many secondary hydrogens are present in the hydrocarbon below? CH3 CH3 16 Hydrocarbon chains are formed by a series of bonds between carbon atoms. These chains may be long or short: for instance, ethane contains just two carbons in a row, while decane contains ten. Not all hydrocarbons are straight chains. For example, while decane’s ten carbon atoms are lined up in a row, other hydrocarbons with the same molecular
Source Image: numerade.com
Download Image
Hydrocarbons – Types, Classification and Properties – WBBSE Solutions Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4 ), carbon forms covalent bonds with four hydrogen atoms. Each bond corresponds to a pair of shared electrons (one from carbon and one from hydrogen), giving carbon the eight electrons it needs for a full outer shell.
Source Image: wbbsesolutions.guide
Download Image
Solved Question 19 How many secondary hydrogens are present | Chegg.com Oct 12, 2023The term ‘ secondary hydrogens ‘ generally refers to hydrogen atoms that are attached to secondary carbon atoms, which, in this case, are those attached to two other carbon atoms. The molecular formula of this compound is C8H16.
Source Image: chegg.com
Download Image
Organic Compounds
Solved Question 19 How many secondary hydrogens are present | Chegg.com Oct 27, 2022This compound contains 16 hydrogen atoms for a molecular formula of C 8 H 16. Location of the hydrogen atoms: In this figure a ring composed of four C H subscript 2 groups and one C H group in a pentagonal shape is shown. From the C H group, which is at the right side of the pentagon, a C H is bonded.
in the given compound : Hydrocarbons – Types, Classification and Properties – WBBSE Solutions Hydrocarbon chains are formed by a series of bonds between carbon atoms. These chains may be long or short: for instance, ethane contains just two carbons in a row, while decane contains ten. Not all hydrocarbons are straight chains. For example, while decane’s ten carbon atoms are lined up in a row, other hydrocarbons with the same molecular