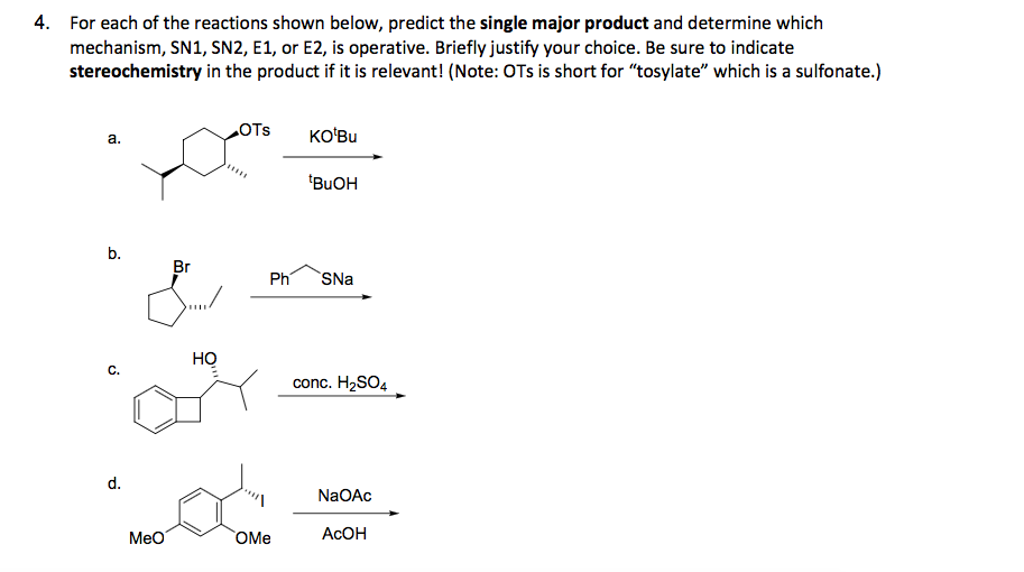
Sn1, Sn2, E1, and E2 reactions form the basis for understanding why certain products are more likely to form than others. We will learn about the reaction mechanisms, and how nucleophilicity and electrophilicity can be used to choose between different reaction pathways. … Sn1, Sn2, E1, and E2 reactions form the basis for understanding why
The SN1 Reaction Mechanism – Master Organic Chemistry
If the carbon is primary, then rule out S N 1 and E1 unless the carbocation can be resonance-stabilized. If the carbon is tertiary, then rule out S N 2. (3) Construct the following table, and for each factor, add a check mark to the column for the reaction that is favored. (4) The column with the most check marks is the winner.
Source Image: chegg.com
Download Image
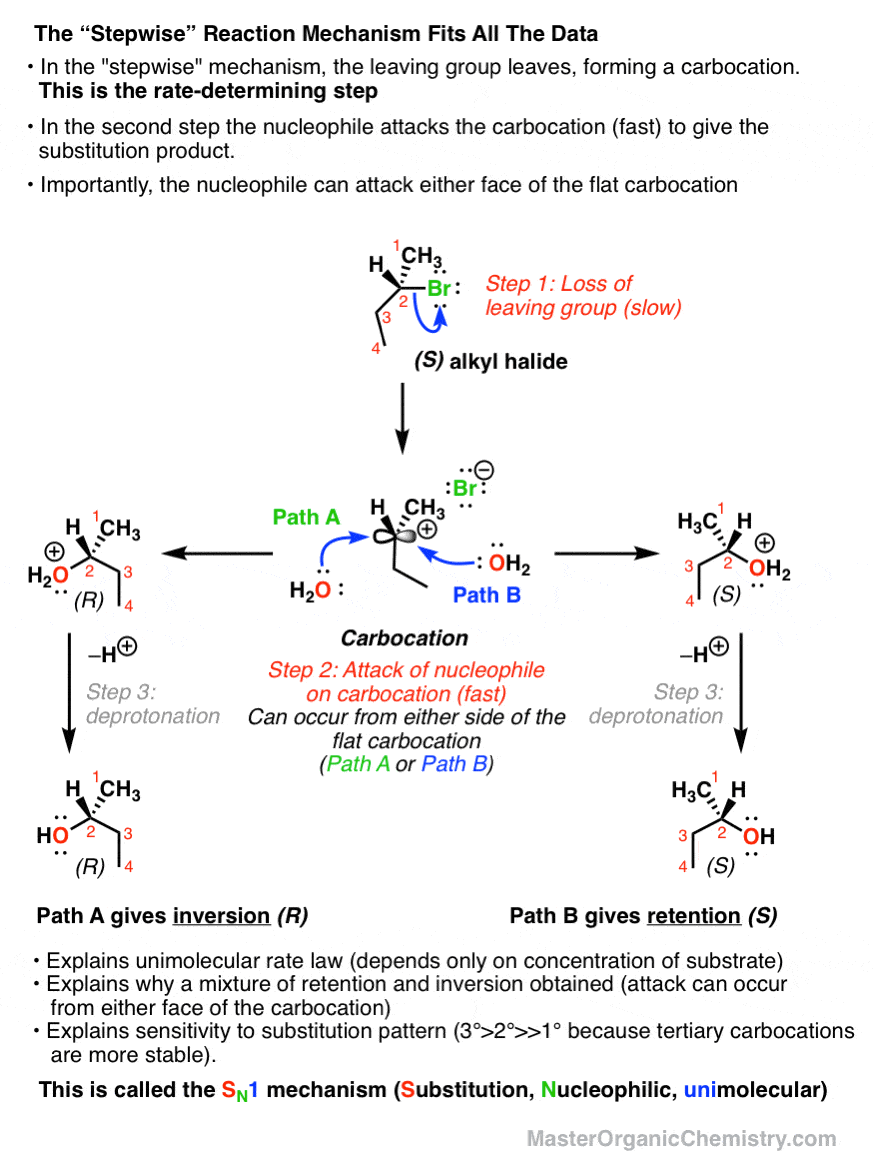
The SN1 Reaction Mechanism. There are two important classes of nucleophilic substitution mechanisms – the SN1 and SN2 mechanisms.; The SN1 mechanism is distinct from the SN2 in several different ways. The reaction is fastest for tertiary alkyl halides and slowest for primary (and methyl) halides The rate law is unimolecular – it is only dependent on the concentration of substrate (i.e

Source Image: pearson.com
Download Image
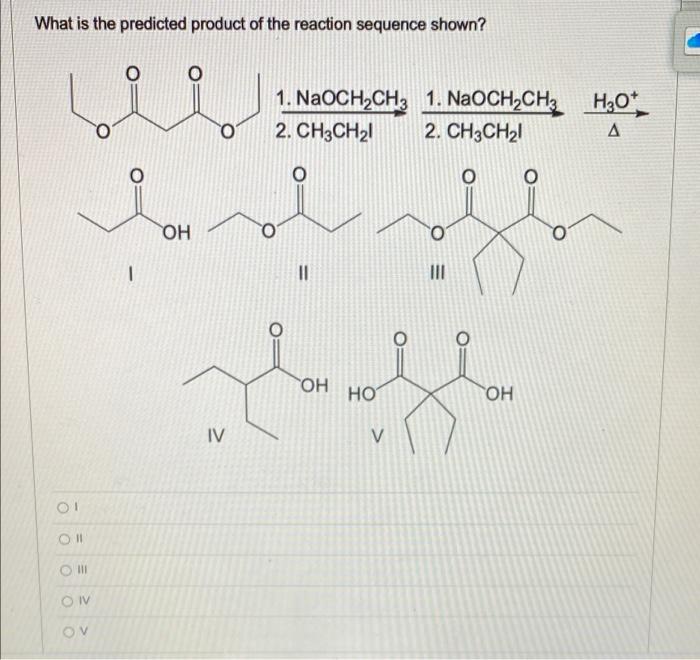
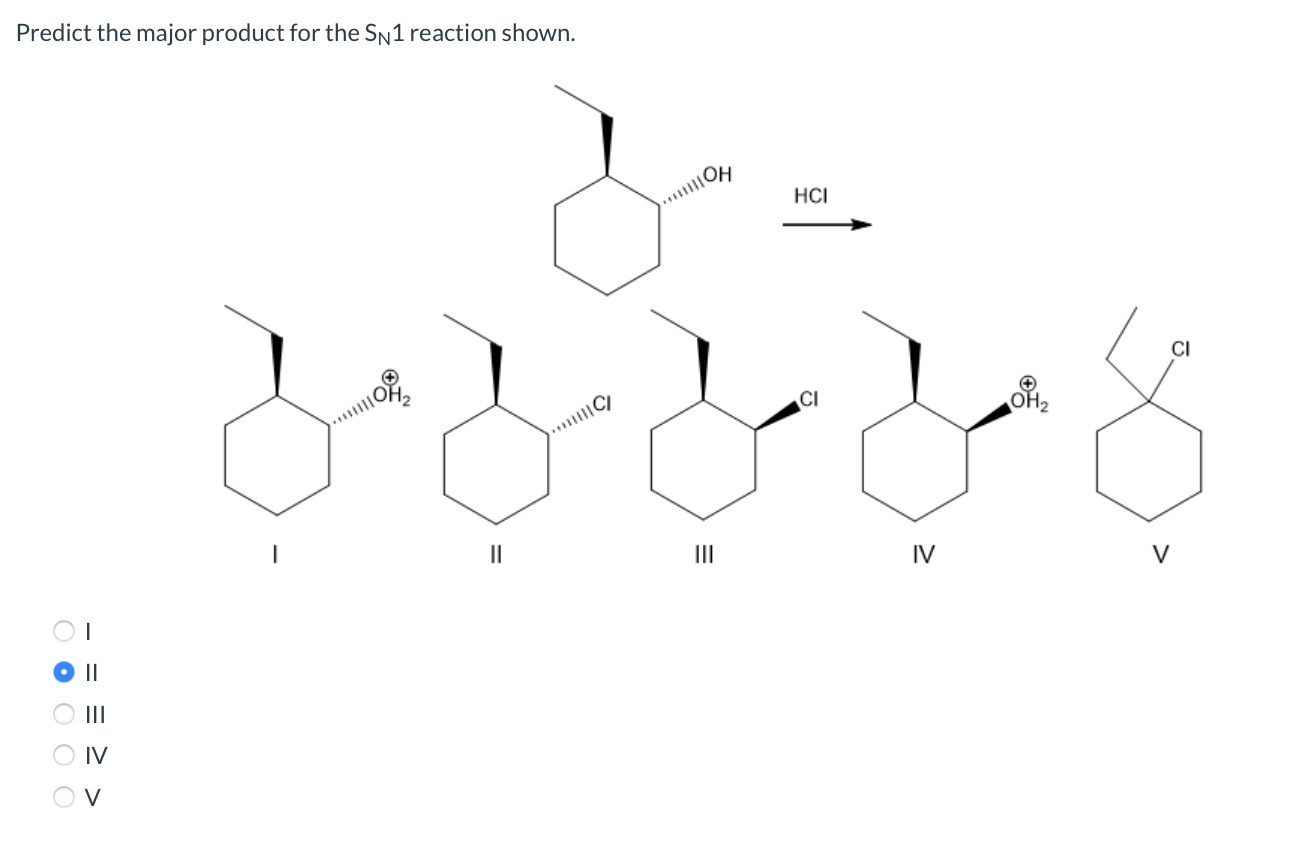
Solved Predict the product for the following SN1 reaction. | Chegg.com Lesson 1: SN1. Identifying nucleophilic and electrophilic centers. SN1 mechanism: Kinetics and substrates. Sn1 mechanism: stereochemistry. Carbocation stability: Recap. Effect of substrate on the rate of an SN1 reaction– Part 1. Effect of substrate on the rate of SN1 reaction-Part 2. Identifying the major product– Carbocation rearrangement.
Source Image: chegg.com
Download Image
What Are The Predicted Products For The Sn1 Reaction Shown
Lesson 1: SN1. Identifying nucleophilic and electrophilic centers. SN1 mechanism: Kinetics and substrates. Sn1 mechanism: stereochemistry. Carbocation stability: Recap. Effect of substrate on the rate of an SN1 reaction– Part 1. Effect of substrate on the rate of SN1 reaction-Part 2. Identifying the major product– Carbocation rearrangement. Leaving Group Effect on S N 1. As is the case for the S N 2 reaction, a good leaving group is also required for the S N 1 mechanism, and all the discussions we had in section 7.3 still apply.. Nucleophile Unlike an S N 2 reaction, the rate-determining step of the S N 1 reaction does not include nucleophiles, so theoretically the strength of a nucleophile has no effect on the S N 1 reaction.
Solved What is the predicted product of the reaction | Chegg.com
This page titled 7.12: The SN1 Mechanism is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Layne Morsch. A second model for a nucleophilic substitution reaction is called the ‘dissociative’, or ‘SN1’ mechanism: in this picture, the C-X bond breaks first, before the nucleophile approaches. SN1 Reaction Mechanism in Nucleophilic Substitutnions | Inductive effect, Rate equation, Energy activities

Source Image: in.pinterest.com
Download Image
Solved Predict the major product for the Sn1 reaction shown. | Chegg.com This page titled 7.12: The SN1 Mechanism is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Layne Morsch. A second model for a nucleophilic substitution reaction is called the ‘dissociative’, or ‘SN1’ mechanism: in this picture, the C-X bond breaks first, before the nucleophile approaches.
Source Image: chegg.com
Download Image
The SN1 Reaction Mechanism – Master Organic Chemistry Sn1, Sn2, E1, and E2 reactions form the basis for understanding why certain products are more likely to form than others. We will learn about the reaction mechanisms, and how nucleophilicity and electrophilicity can be used to choose between different reaction pathways. … Sn1, Sn2, E1, and E2 reactions form the basis for understanding why
Source Image: masterorganicchemistry.com
Download Image
Solved Predict the product for the following SN1 reaction. | Chegg.com The SN1 Reaction Mechanism. There are two important classes of nucleophilic substitution mechanisms – the SN1 and SN2 mechanisms.; The SN1 mechanism is distinct from the SN2 in several different ways. The reaction is fastest for tertiary alkyl halides and slowest for primary (and methyl) halides The rate law is unimolecular – it is only dependent on the concentration of substrate (i.e
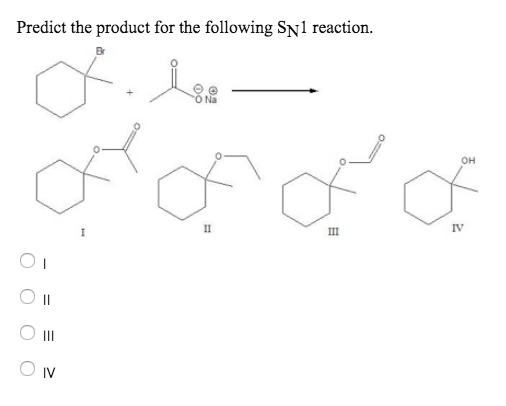
Source Image: chegg.com
Download Image
7.11 SN2 reaction problem solving with predict the products type questions – YouTube In an SN2 reaction, the rate law is 2nd order. That is, the reaction rate depends on the concentrations of two components, the alkyl halide and the nucleophile. Hence the term Substitution Nucleophilic 2nd order. The 1 in SN1 and the 2 in SN2 come from the kinetics of the reactions, not from 1° and 2°. Hope this helps.

Source Image: youtube.com
Download Image
Solved What are the predicted products for the SN1 reaction | Chegg.com Lesson 1: SN1. Identifying nucleophilic and electrophilic centers. SN1 mechanism: Kinetics and substrates. Sn1 mechanism: stereochemistry. Carbocation stability: Recap. Effect of substrate on the rate of an SN1 reaction– Part 1. Effect of substrate on the rate of SN1 reaction-Part 2. Identifying the major product– Carbocation rearrangement.
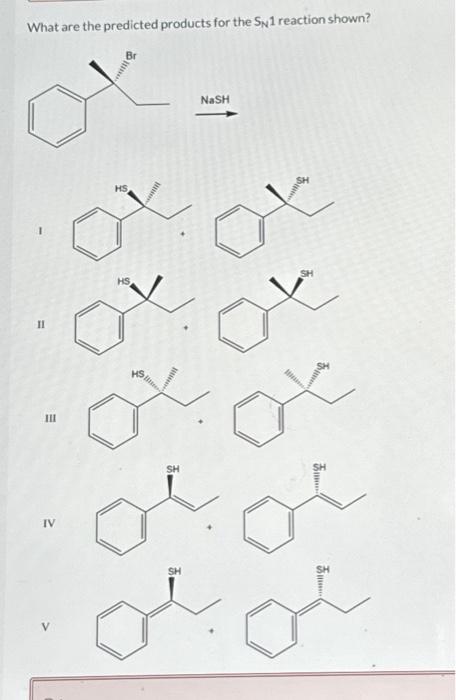
Source Image: chegg.com
Download Image
Solved What are the predicted products for the SN1 reaction | Chegg.com Leaving Group Effect on S N 1. As is the case for the S N 2 reaction, a good leaving group is also required for the S N 1 mechanism, and all the discussions we had in section 7.3 still apply.. Nucleophile Unlike an S N 2 reaction, the rate-determining step of the S N 1 reaction does not include nucleophiles, so theoretically the strength of a nucleophile has no effect on the S N 1 reaction.
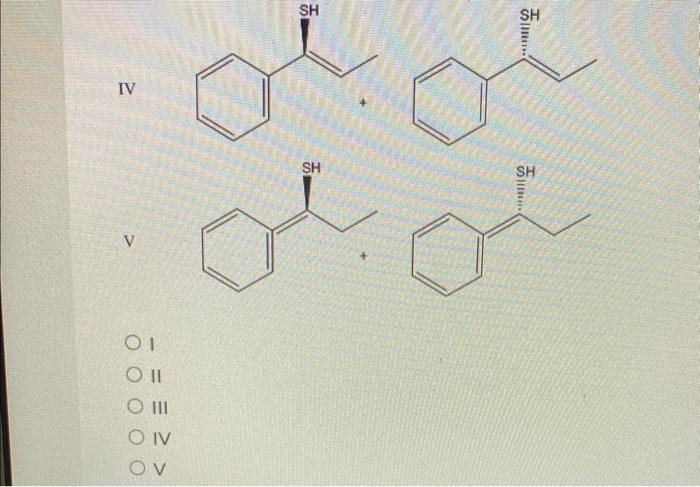
Source Image: chegg.com
Download Image
Solved Predict the major product for the Sn1 reaction shown. | Chegg.com
Solved What are the predicted products for the SN1 reaction | Chegg.com If the carbon is primary, then rule out S N 1 and E1 unless the carbocation can be resonance-stabilized. If the carbon is tertiary, then rule out S N 2. (3) Construct the following table, and for each factor, add a check mark to the column for the reaction that is favored. (4) The column with the most check marks is the winner.
Solved Predict the product for the following SN1 reaction. | Chegg.com Solved What are the predicted products for the SN1 reaction | Chegg.com In an SN2 reaction, the rate law is 2nd order. That is, the reaction rate depends on the concentrations of two components, the alkyl halide and the nucleophile. Hence the term Substitution Nucleophilic 2nd order. The 1 in SN1 and the 2 in SN2 come from the kinetics of the reactions, not from 1° and 2°. Hope this helps.